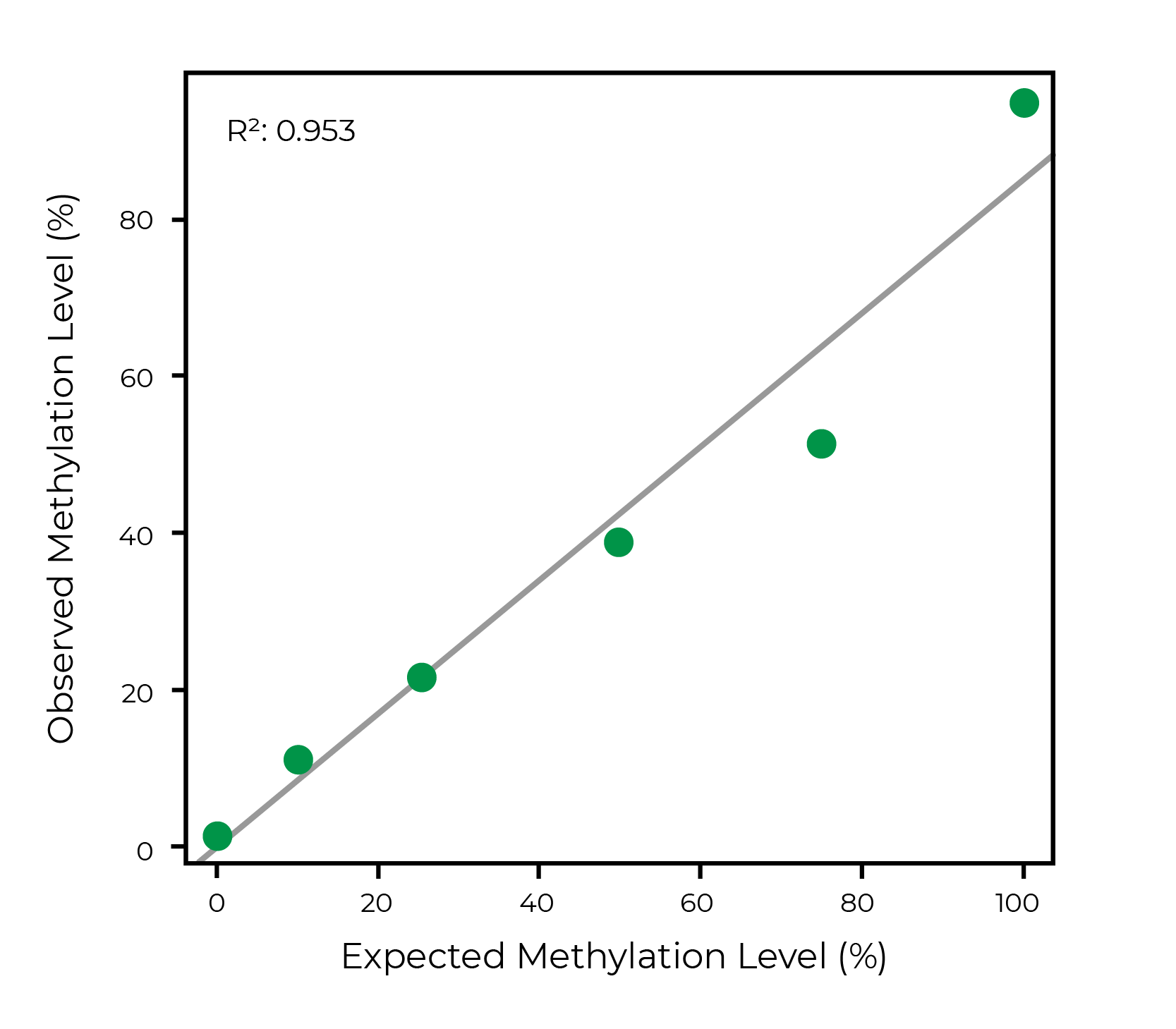
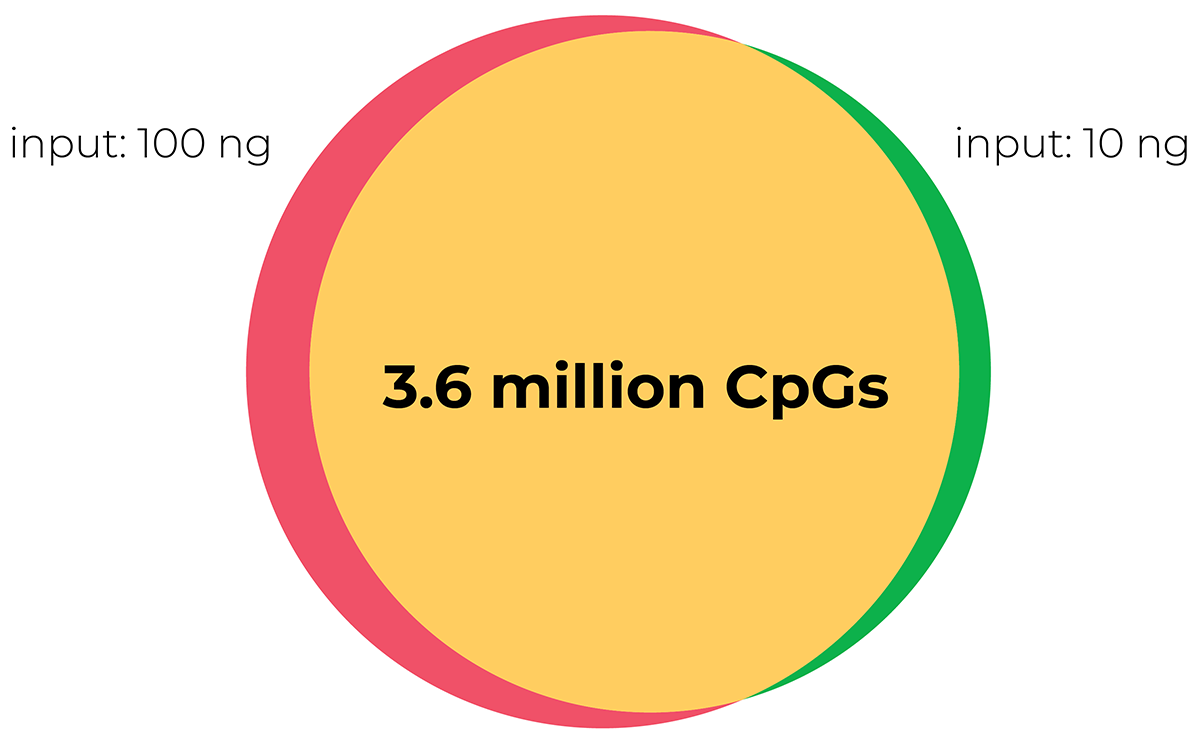
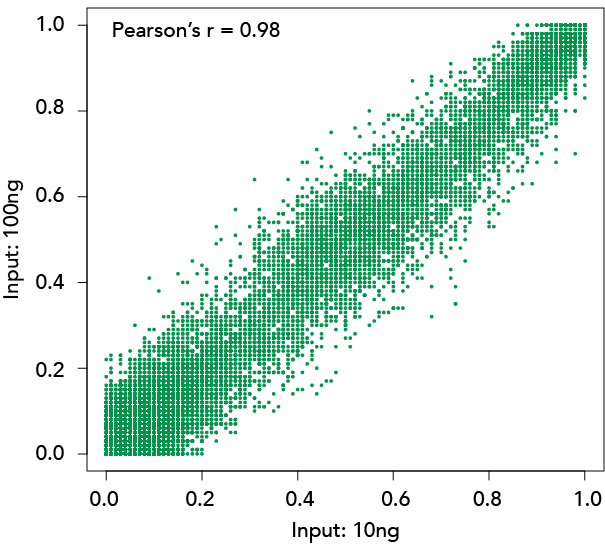
Lu, Y. et al. (2020) Reprogramming to recover youthful epigenetic information and restore vision. Nature 588, 124–129 (2020)
Wendell P. et al. (2020) A cost-effective approach to DNA methylation detection by Methyl Sensitive DArT sequencing. PLoS ONE 15(6): e0233800.
https://doi.org/10.1371/journal.pone.0233800
Researchers study epigenetic regulation on myonuclear utilizing RRBS in response to acute hypertrophic stimulus. Von Walden F. et al. discovered 655 hypomethylated and 84 hypermethylated sites in promoters from top 3000 differential methylation sites. Pathway analysis was done on differential methylation promoter regions revealed robust hypomethylation sites were influence on regulator of skeletal muscle growth, autophagy and ribosome biogenesis.
Von Walden F. et al. (2020) The Myonuclear DNA Methylome in Response to an Acute Hypertrophic Stimulus. Epigenetics. April, 2020 DOI: 10.1080/15592294.2020.1755581
Beetch M. et al. study DNA methylation pattern of different stages of breast cancer in canine to help define triple-negative in situ (DCIS) progression. Methyl-Miniseq® is utilized to discover 68 out of 128 and 96 out of 150 gene with differentially methylated promoters specific to DCIS and invasive ductal carcinoma, respectively. Differential methylated genes were related to transcriptional regulation, apoptosis, signal transduction and cell migration.
Beetch, M., Harandi-Zadeh, S., Yang, T. et al. DNA methylation landscape of triple-negative ductal carcinoma in situ (DCIS) progressing to the invasive stage in canine breast cancer. Sci Rep 10, 2415 (2020). https://doi.org/10.1038/s41598-020-59260-4
Littlejohn, B. et al. Influence of prenatal transportation stress-induced differential DNA methylation on the physiological control of behavior and stress response in suckling Brahman bull calves, Journal of Animal Science, Volume 98, Issue 1, January 2020, skz368, https://doi.org/10.1093/jas/skz368
Zhang Q et al. (2019) Dynamic Changes in Gene Mutational Landscape With Preservation of Core Mutations in Mantle Cell Lymphoma Cells. Front. Oncol. 9:568. doi: 10.3389/fonc.2019.00568
González, B. et al. (2019). Folate Metabolism Interferes Plant Immunity through 1C Methionine Synthase-directed Genome-wide DNA Methylation Enhancement. Molecular Plant. doi:10.1016/j.molp.2019.04.013
Bacon, E. R. et al. (2019). Neuroendocrine aging precedes perimenopause and is regulated by DNA methylation. Neurobiology of Aging, 74, 213–224. doi:10.1016/j.neurobiolaging.2018.09.029
Littlejohn, B. P. et al. (2018). Prenatal transportation stress alters genome-wide DNA methylation in suckling Brahman bull calves. Journal of Animal Science. doi:10.1093/jas/sky350
Arechederra, M. et al. (2018). Hypermethylation of gene body CpG islands predicts high dosage of functional oncogenes in liver cancer. Nature Communications, 9(1). doi:10.1038/s41467-018-05550-5
Mishra, R. et al. (2018). Stromal epigenetic alterations drive metabolic and neuroendocrine prostate cancer reprogramming. J Clin Invest, 128(10):4472-4484. doi:10.1172/JCI99397
Kaur, G. et al. (2018). Alternative splicing of helicase-like transcription factor (Hltf): Intron retention-dependent activation of immune tolerance at the feto-maternal interface. PLOS ONE, 13(7), e0200211. doi:10.1371/journal.pone.0200211
Zhang, Na. et al. (2018). Elevated hepatic expression of H19 long noncoding RNA contributes to diabetic hyperglycemia. JCI Insight. 3(10):e120304. doi: 10.1172/jci.insight.120304
Ryu, T. et al. (2018). The epigenetic landscape of transgenerational acclimation to ocean warming. Nature Climate Change, 8(6), 504–509. doi:10.1038/s41558-018-0159-0
Jorgensen, B.G. et al. DNA methylation, through DNMT1, has an essential role in the development of gastrointestinal smooth muscle cells and disease. Cell Death Dis 9, 474 (2018). doi:10.1038/s41419-018-0495-z
Verma, D. et al. (2018). Genome-Wide DNA Methylation Profiling Identifies Differential Methylation in Uninvolved Psoriatic Epidermis. Journal of Investigative Dermatology, 138(5), 1088–1093. doi:10.1016/j.jid.2017.11.036
Zhang, Z. et al. Structural basis for DNMT3A-mediated de novo DNA methylation. Nature 554, 387–391 (2018). doi:10.1038/nature25477
Waters, S. A. et al. (2017). Landscape of DNA Methylation on the Marsupial X. Molecular Biology and Evolution, 35(2), 431–439. doi:10.1093/molbev/msx297
Yaish, M. W. et al. (2018). Genome-wide DNA Methylation analysis in response to salinity in the model plant caliph medic (Medicago truncatula). BMC Genomics, 19(1). doi:10.1186/s12864-018-4484-5
Xie, K. et al. (2018). Epigenetic alterations in longevity regulators, reduced life span, and exacerbated aging-related pathology in old father offspring mice. Proceedings of the National Academy of Sciences, 115(10), E2348–E2357. doi:10.1073/pnas.1707337115
Al-Harrasi, I. et al. (2018). Differential DNA methylation and transcription profiles in date palm roots exposed to salinity. PLOS ONE, 13(1), e0191492. doi:10.1371/journal.pone.0191492
Jones, C. M. et al. (2018). Genome-Wide Characterisation of DNA Methylation in an Invasive Lepidopteran Pest, the Cotton Bollworm Helicoverpa armigera . G3:Genes|Genomes|Genetics, g3.1112.2017. doi:10.1534/g3.117.1112
McCormick, H. et al. (2017) Isogenic mice exhibit sexually-dimorphic DNA methylation patterns across multiple tissues. BMC Genomics 18, 966. https://doi.org/10.1186/s12864-017-4350-x
Bach, A. et al. (2017). Consequences of supplying methyl donors during pregnancy on the methylome of the offspring from lactating and non-lactating dairy cattle. PLOS ONE, 12(12), e0189581. doi:10.1371/journal.pone.0189581
Rea, M. et al. (2018). Selective inhibition of CTCF binding by iAs directs TET-mediated reprogramming of 5-hydroxymethylation patterns in iAs-transformed cells. Toxicology and Applied Pharmacology, 338, 124–133. doi:10.1016/j.taap.2017.11.015
Dworkin, M. et al. (2017)Analyses of methylomes of upland and lowland switchgrass (Panicum virgatum) ecotypes using MeDIP-seq and BS-seq. BMC Genomics 18, 851. doi:10.1186/s12864-017-4218-0
Ellison, E.M. et al. (2017) Single-Base Resolution Mapping of 5-Hydroxymethylcytosine Modifications in Hippocampus of Alzheimer’s Disease Subjects. J Mol Neurosci 63, 185–197. doi:10.1007/s12031-017-0969-y
Moghadam, H.K. et al. (2017) Impacts of Early Life Stress on the Methylome and Transcriptome of Atlantic Salmon. Sci Rep 7, 5023. doi:10.1038/s41598-017-05222-2
Mason, A.G. et al. (2017) SMCHD1 regulates a limited set of gene clusters on autosomal chromosomes. Skeletal Muscle 7, 12. doi:10.1186/s13395-017-0129-7
Korkmaz, F.T. et al. (2017) Genome-wide methylation analysis reveals differentially methylated loci that are associated with an age-dependent increase in bovine fibroblast response to LPS. BMC Genomics 18, 405. doi:10.1186/s12864-017-3796-1
Begue, G. et al. (2017). DNA methylation assessment from human slow- and fast-twitch skeletal muscle fibers. Journal of Applied Physiology, 122(4), 952–967. doi:10.1152/japplphysiol.00867.2016
Nagymihály, M. et al. (2017). Ploidy-dependent changes in the epigenome of symbiotic cells correlate with specific patterns of gene expression. Proceedings of the National Academy of Sciences, 114(17), 4543–4548. doi:10.1073/pnas.1704211114
Rea, M. et al. (2017) Genome-wide DNA methylation reprogramming in response to inorganic arsenic links inhibition of CTCF binding, DNMT expression and cellular transformation. Sci Rep 7, 41474. https://doi.org/10.1038/srep41474
Mutoji, K. et al. (2016). TSPAN8 Expression Distinguishes Spermatogonial Stem Cells in the Prepubertal Mouse Testis. Biology of Reproduction, 95(6), 117–117. doi:10.1095/biolreprod.116.144220
Doherty, et al. (2016) The CD4+ T cell methylome contributes to a distinct CD4+ T cell transcriptional signature in Mycobacterium bovis-infected cattle. Sci Rep 6, 31014 (2016). doi:10.1038/srep31014
Zhong, et al. (2017) Metformin alters DNA methylation genome-wide via the H19/SAHH axis. Oncogene 36, 2345–2354 (2017). doi:10.1038/onc.2016.391
Trella, et al. (2016). Genome-wide analysis identifies differential promoter methylation of Leprel2 , Foxf1 , Mmp25, Igfbp6 , and Peg12 in murine tendinopathy. Journal of Orthopaedic Research, 35(5), 947–955. doi:10.1002/jor.23393
Crampton, M. et al. (2016). Analyses of Methylomes Derived from Meso-American Common Bean (Phaseolus vulgaris L.) Using MeDIP-Seq and Whole Genome Sodium Bisulfite-Sequencing. Frontiers in Plant Science, 7. doi:10.3389/fpls.2016.00447
Barua, S. et al. (2016). DNA Methylation Profiling at Single-Base Resolution Reveals Gestational Folic Acid Supplementation Influences the Epigenome of Mouse Offspring Cerebellum. Frontiers in Neuroscience, 10. doi:10.3389/fnins.2016.00168
Steyaert, S. et al. (2016) A genome-wide search for epigenetically regulated genes in zebra finch using MethylCap-seq and RNA-seq. Sci Rep 6, 20957. doi:10.1038/srep20957
Zhou, J. et al. (2015) H19 lncRNA alters DNA methylation genome wide by regulating S-adenosylhomocysteine hydrolase. Nat Commun 6, 10221. doi:10.1038/ncomms10221
Weyrich, A. et al. (2015). Paternal intergenerational epigenetic response to heat exposure in male Wild guinea pigs. Molecular Ecology, 25(8), 1729–1740. doi:10.1111/mec.13494
Kozlenkov, A. et al. (2015). Substantial DNA methylation differences between two major neuronal subtypes in human brain. Nucleic Acids Research, 44(6), 2593–2612. doi:10.1093/nar/gkv1304
Study authors investigated DNA methylation changes in mouse skeletal muscle following a 4 week regimen of regular exercise. Using Zymo Research’s Methyl-MiniSeq platform, Kanzleiter et al. detected 3692 differentially methylated CpGs distributed among 2762 promoter regions. When coupled with gene expression profiles, integrated analysis revealed 361 genes in which changes of DNA methylation were associated with changes in gene expression. These genes included those related to muscle growth and differentiation as well as metabolic regulation.
Kanzleiter T, et al. (2015) Exercise training alters DNA-methylation patterns in genes related to muscle growth and differentiation in mice. Am J Physiol Endocrinol Metab. 15;308(10):E912-E920. doi: 10.1152
Researchers sought to better understand epigenetic changes that occur in hepatic stellate cells (HSCs) upon activation. Götze, et al. stimulated HSC activation by altering in vitro culture conditions. The genome-wide DNA methylation patterns were then determined using the Methyl-MiniSeq service from Zymo Research. The data revealed approximately 400 differentially methylated regions and, according to DAVID pathway analysis, contained genes enriched for processes relevant during HSC activation. Furthermore, locus-specific validation tests revealed that DNA methylation changes correlated with gene expression changes of select genes. The researchers went on to show that the DNA methylation changes are likely the result of an active, as opposed to passive, demethylation mechanism.
Götze, S., et al. (2015) Epigenetic changes during hepatic stellate cell activation. PLOS One. June 12, 2015. DOI: 10.1371/journal.pone.0128745
Researchers investigated how alcohol affects the regulatory mechanisms needed for normal stem cell differentiation and embryonic development. Study authors utilized genome-wide DNA methylation analysis services provided by Zymo Research to help characterize epigenetic and gene expression changes in human embryonic stem cells (hESCs) exposed to low amounts of alcohol (Ethanol, EtOH). Genome-wide analysis revealed that alcohol exposure caused a significant amount of regional promoter hypermethylation, especially affecting segments of chromosomes 2, 16, and 18, and differential expression in undifferentiated hESCs versus controls. The study authors also found that EtOH exposure affected key pathways involved in normal cell function, such as metabolism, and reduced hESC pluripotency.
Khalid, O, et al. (2014) Gene expression signatures affected by alcohol-induced DNA methylomic deregulation in human embryonic stem cells. Stem Cell Research. 12(3): 791-806.
Researchers investigated the effects of di-(2-ethylhexyl) phthalate (DEHP), a ubiquitous plasticizer and a known endocrine disruptor, on DNA methylation changes in the adrenal gland of adult male rats exposed to DEHP in utero. Using the Methyl-MiniSeq™ service from Zymo Research, study authors found 972 differentially methylated CpGs out of 2,183,479 CpG sites surveyed throughout the genome. Most differentially methylated CpGs were found within CpG islands (40%). Interestingly, the second highest frequency of differentially methylated CpGs were found within shore/shelf regions (30%). By contrast, promoter regions did not contain a significant number of differentially methylated CpGs, suggesting that DEHP targets epigenomic regions that regulate tissue-specific expression. The researchers also identified several hotspots of differentially methylated CpGs that coincided with regions of differentially expressed genes. Two such hotspots at chr20p12 correlated with deregulation of gene expression. That region contains genes critical for the proper function of the immune system. The study’s findings support the notion that the typical amounts of DEHP present in the environment may influence a significant number of regions across the epigenome.
Martinez-Arguelles, DB & Papadopoulos, V. (2014) Identification of Hot Spots of DNA Methylation in the Adult Male Adrenal in Response to In Utero Exposure to the Ubiquitous Endocrine Disruptor Plasticizer Di-(2-ethylhexyl) Phthalate. Endocrinology. 1(1):124-33.
Researchers utilized Zymo Research’s Methyl MiniSeq and Targeted Bisulfite Sequencing services to identify and validate methylation markers linked to colorectal cancer (CRC) in African Americans. Genome-wide methylation analysis with Methyl MiniSeq revealed 355 differentially methylated CpG sites in 13 genes in addition to hypomethylated Long INterspersed Elements (LINEs) in CRC samples. Furthermore, six of these genes contained the 50 CpGs with the highest degrees of differential methylation and were then studied further using 42 samples and Targeted Bisulfite Sequencing. With the larger sample set and more focused sequencing, the authors were able to validate four genes, EID3, BMP3, GAS7, and GPR75, as novel CRC methylation markers in African American patients.
Ashktorab, H, et al. (2014) DNA methylome profiling identifies novel methylated genes in African American patients with colorectal neoplasia. Epigenetics. 9(4): 503-512
The Methyl-MiniSeq service was used by the authors to determine the role DNA methylation has in stabilizing gene expression of embryonic stem cells transitioning between cellular states. The expression of a pluripotent marker, Rex1, was found to be correlated with the expression of key methylation factors: Dnmt3b, Tet1, and Prdm14. Methyl-MiniSeq data showed that cells in the Rex1-low state had higher levels of promoter methylation compared to those in the Rex1-high state. Differential methylation occurred at the promoters of key ESC regulators such as Esrrb, Tet1, and Tcl2; these results correlated with the differential gene expression observed by single-molecular RNA-FISH (smFISH). Furthermore, cells with DNA methyltransferase knock-outs or those treated with a DNA methylation inhibitor had fewer cells in the Rex1-low state. These findings suggest that methylation is required for the transition between two cellular states and its maintenance.
Singer, Z, et al. (2014) Dynamic heterogeneity and DNA methylation in embryonic stem cells. Mol Cell. 55(2): 319-331. doi: 10.1016/j.molcel.2014.06.029.
Researchers examining the epigenetic modifications in oral keratinocytes following Epstein-Barr virus (EBV) infection used an expanded and streamlined version of Reduced Representation Bisulfite Sequencing and bioinformatics analysis (Methyl-MiniSeq). The group found that EBV infection induced long-lasting DNA methylation changes at CpG islands, promoters, and within gene bodies when compared to uninfected cells. Furthermore, the DNA methylation changes correlated with gains and losses in expression of a subset of affected genes. The findings provide mechanistic insights as to how EBV infection contributes to DNA methylation changes commonly observed in EBV-associated carcinomas and hints at the potential for future epigenetics-based therapies.
Birdwell CE, et al. (2014) Genome-wide DNA methylation as an epigenetic consequence of Epstein-Barr virus infection of immortalized keratinocytes. J Virol. pii:JVI.00972-14.
The Methyl-MiniSeq (expanded-RRBS) service from Zymo Research was used to study how disturbed blood flow (d-flow) epigenetically regulates endothelial gene expression and causes endothelial cells to initiate and sustain the development of plaque that contributes to atherosclerosis. Using the Methyl-MiniSeq platform, the researchers found that sections of carotid artery exposed to d-flow exhibited significantly higher levels of hypermethylated CpG sites compared to sections of carotid artery not exposed to d-flow. The method also revealed that treating mice with the DNA methyltransferase (DNMT)-inhibitor 5Aza reduced the number of hypermethylated CpG sites in endothelial sections exposed to d-flow. These findings suggest that DNMT is the mechanism by which d-flow induces genome-wide DNA methylation changes in endothelial cells.
Dunn J, et al. (2014) Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest. 124(7):3187-3199. doi: 10.1172/JCI74792.
Using the Methyl-MiniSeq service, study authors investigated how the BCL6 oncogene contributes to diffuse large B-cell lymphoma (DLBCL) development. The investigators used a mouse model in which Bcl6 expression was restricted to hematopoietic stem/progenitor cells (HSPCs) and found that BCL6 predisposed developing B-cells to a malignant fate. Data generated via the Methyl-MiniSeq service helped identify epigenetically reprogrammed DNA methylation patterns in HSPC genomes. The proposed “hit-and-run” model for BCL6 driven tumor development suggests a possible reason as to why certain treatment strategies fail in some lymphoma patients and not others and suggests new avenues for potential therapeutic development.
Green M, et al. (2014) Transient expression of Bcl6 is sufficient for oncogenic function and induction of mature B-cell lymphoma. Nature Communications. 5:3904. doi: 10.1038/ncomms4904.
The study’s authors used Zymo’s Methyl-MiniSeq service to identify genome-wide DNA methylation pattern changes in the brain tissues of mouse offspring whose pregnant mothers were fed a diet 10 times higher in folic acid concentration compared to low folic acid controls. The researchers found significant changes in the global patterns of DNA methylation between the two groups of mouse pups. Furthermore, the study showed significant changes in the levels of methylated cytosines in both CpG as well as CHG and CHH contexts in CpG islands, gene promoters, gene bodies, and non-coding regions at single-base resolution. In many cases the DNA methylation changes correlated with differential gene expression as measured by quantitative real-time PCR. The findings suggest that maternal folic acid supplementation may profoundly influence methylation and developmental patterns in children and has substantial implications for the observed recent increase in diseases such as Autism Spectrum Disorder.
Barua S, et al. (2014) Single-base resolution of mouse offspring brain methylome reveals epigenome modifications caused by gestational folic acid. Epigenetics Chromatin. 7(1):3. doi: 10.1186/1756-8935-7-3.
The Methyl-MiniSeq® (expanded RRBS) Service from Zymo Research was used to investigate genome-wide changes in DNA methylation in mouse medulloblastoma cancer models. The authors detected relatively few cancer-specific regions that exhibited DNA hypermethylation, which was different from what was previously observed for human cancers. The researchers next investigated genome-wide DNA methylation patterns in mouse models for additional types of human cancers, including breast cancer Burkitt’s lymphoma. They found different methylation patterns in the mouse models than their human counterparts, suggesting that some types of cancers require development of better animal models to use for studies related to cancer diagnostics and therapeutics.
Diede SJ, et al. (2013) Fundamental differences in promoter CpG island DNA hypermethylation between human cancer and genetically engineered mouse models of cancer. Epigenetics. 8(12): 1254-1260.
Researchers used the Methyl-MiniSeq® Service from Zymo Research to investigate the mechanisms responsible for establishment of rhabdomyosarcoma (RMS) tumors in children. Rhabdomyosarcomas are pediatric skeletal muscle cancers that form due to incomplete differentiation of muscle cells. MyoD is a cellular factor that generally drives the differentiation of normal skeletal muscle cells, but is not able to do so in RMS cells even though it is expressed to normal levels. In this report, the authors used Methyl-MiniSeq® to demonstrate that one of the MyoD target genes responsible for muscle differentiation, JDP2, is not expressed due to DNA hypermethylation in its promoter region, and that this epigenetic silencing likely contributes to the lack of differentiation in rhabdomyosarcoma cells.
MacQuarrie KL, et al. (2013) Comparison of genome-wide binding of MyoD in normal human myogenic cells and rhabdomyosarcomas identifies regional and local suppression of promyogenic transcription factors. Mol Cell Biol. 33(4):773-84.
Researchers used the Methyl-MiniSeq® Service from Zymo Research to investigate the DNA methylation profiles of a mouse model for multiple myeloma. Compared to wild type controls, mouse hematopoietic stem/progenitor cells ectopically expressing the MafB oncogene underwent specific epigenetic reprogramming that predisposed activated plasma cells to a cancerous fate. The findings suggest a novel MafB-mediated "hands off" epigenetic molecular mechanism for multiple myeloma and associated plasma tumor cell initiation, and provide insight for innovative, targeted cancer therapy.
Vicente-Dueñas C, et al. (2012) A novel molecular mechanism involved in multiple myeloma development revealed by targeting MafB to haematopoietic progenitors. EMBO J. 31(18):3704-17.