Transfecting mammalian cells with plasmid DNA is a critical tool for studying biological processes, developing therapeutics, gene editing, producing proteins, and generating recombinant viruses. In addition, modern DNA synthesis technologies have simplified the construction of variant gene libraries for evaluating candidates of interest with transfection. However, plasmid DNA that meets the quantity, concentration, and low endotoxin requirements for mammalian cell transfection typically uses slow gravity flow anion-exchange columns and lengthy alcohol precipitation steps, making the process poorly suited for high-throughput and automated processing.
Zymo Research’s superior plasmid prep chemistry used with INTEGRA’s pipetting technology is ideal for the high-throughput preparation of ultra-pure, plasmid that is suitable for transfecting mammalian cells. This application note details an automated transfection-grade miniprep protocol that drastically reduces the number of hands-on steps, enabling transfection of up to 96 different constructs in as little as a couple of days.
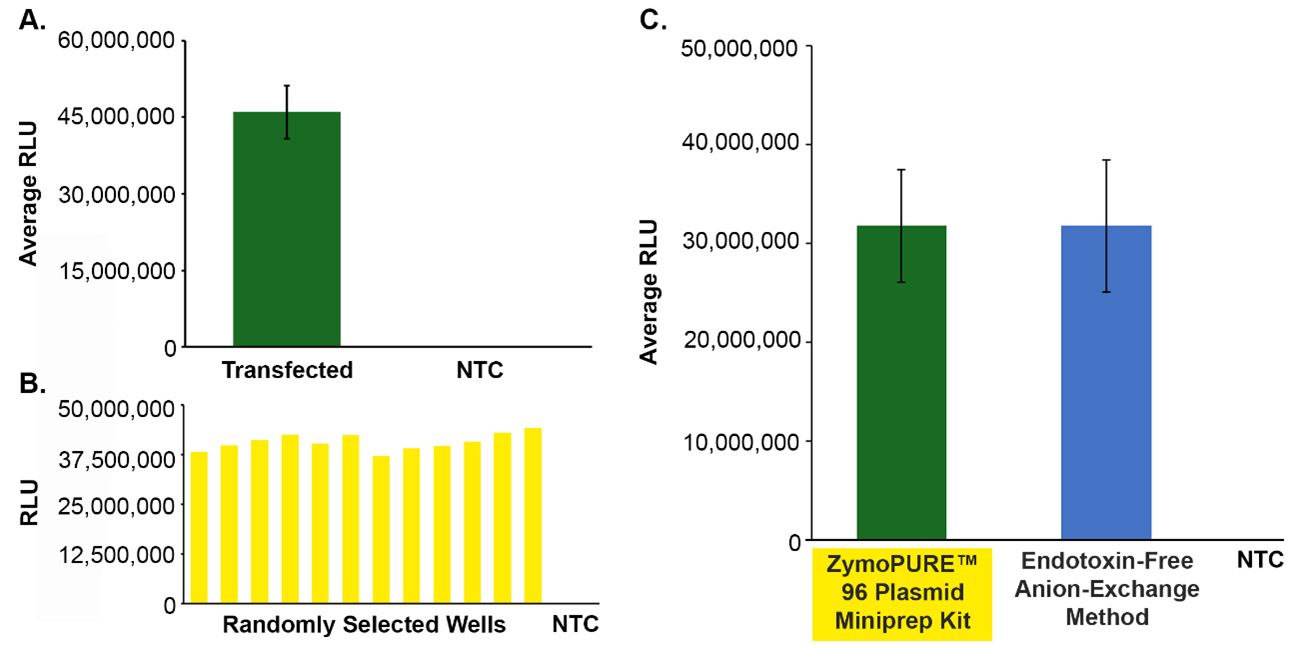
To demonstrate the feasibility of using this technique to automate high-throughput plasmid purification, pGL3 was purified from up to 5 ml of overnight E. coli culture. The purity and yield of the recovered plasmid was assessed using spectrophotometry, whole plasmid sequencing, and mammalian cell transfection. It was found that the plasmid purified using this technique is high-yielding and free from salt, protein, and E. coli host DNA. Additionally, transfection results in consistently high RLUs (relative light units) that are comparable to traditional manual anion-exchange methods for transfection-grade plasmid recovery (Figure 1), demonstrating that this method for high-throughput, transfection-grade plasmid purification is just as effective as tedious manual methods.