Successfully Added to Cart
Customers also bought...
-
 DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the...
DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the... -
 DNA/RNA Shield SafeCollect Swab Collection Kit (1 ml fill) (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA...
DNA/RNA Shield SafeCollect Swab Collection Kit (1 ml fill) (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA...
Highlights
- Efficient: Recovery of ss- or ds- RNA (and DNA) fragments (17-200 nt) from up to 20% (w/v) polyacrylamide gels.
- Concentrated: Up to 10 µg sample in ≥ 6 µl elution.
- Compatible with up to 25% (w/v) polyacrylamide.
Original Manufacturer
Satisfaction 100% guaranteed, read Our Promise
Innovated in California, Made in the USA
Highlights
- Efficient: Recovery of ss- or ds- RNA (and DNA) fragments (17-200 nt) from up to 20% (w/v) polyacrylamide gels.
- Concentrated: Up to 10 µg sample in ≥ 6 µl elution.
- Compatible with up to 25% (w/v) polyacrylamide.
Original Manufacturer
Satisfaction 100% guaranteed, read Our Promise
Innovated in California, Made in the USA
| Cat # | Name | Size | Price | |
|---|---|---|---|---|
| R1003-3-6 | RNA Wash Buffer | 6 ml | $22.00 | |
| C1001-50 | Collection Tubes | 50 Pack | $18.00 | |
| R1060-2-10 | RNA Prep Buffer | 10 ml | $22.00 | |
| R1070-1-10 | RNA Recovery Buffer | 10 ml | $22.00 | |
| R1070-2-20 | RNA MAX Buffer | 20 ml | $65.00 | |
| W1001-1 | DNase/RNase-Free Water | 1 ml | $12.00 | |
| H1001 | Squisher-Single | 10 Pack | $17.00 | |
| C1007-50 | Zymo-Spin IV Columns | 50 Pack | $71.00 | |
Description
Performance
Technical Specifications
| Equipment | Microcentrifuge, 37°C-65°C heat source, dry ice or -80°C freezer |
|---|---|
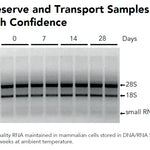
| Purity | RNA is ready for all subsequent analysis and molecular manipulation |
| Sample Source | RNA or DNA fragments (17-200 nt) resolved in PAGE gels (up to 25%), also compatible with TBE-, denatured, urea PAGE gels. Compatible with ethidium bromide or ssRNA-specific dyes (e.g. GelStar). |
| Size Range | RNAs 17-200 nt (fragments 17-28 nt is ≥50 % recovery) |
| Yield | 10 µg RNA (binding capacity), ≥6 µl (elution volume) |
Resources
Documents
FAQ
The recovery rate for fragments 17 to 28 nucleotides is ≥50 %.
Yes, the kit is compatible with both single- or double-stranded RNA (and DNA) fragments.
Yes. Most customer’s do not use PAGE gels for to visualize fragments > 200 nucleotides, but it will be compatible with the kit.
Yes, this kit is compatible with TBE-Urea PAGE gels.
There is no designated upper limit, however it is always more optimal to start with keeping the amount of excised gel as low as possible prior to proceeding with the purification procedure, and scaling up when optimizing gel input amount.
Yes, the kit efficiently removes unincorporated fluorescent dyes, radiolabeled dNTP’s and Biotin.
Citations
Uncleaved RNA transcripts were extracted from denaturing (8.3M ura) 10% polyacrylamide gel using the ZR small-RNA PAGE Recovery kit. This facilitates the generation of ribozyme-based control devices for gene regulatory activities.
Liang JC, et al. A high-throughput, quantitative cell-based screen for efficient tailoring of RNA device activity. Nucleic Acids Research. 2012.The ZR Small-RNA PAGE Recovery kit was used to gel purify short RNAs (50-250 bp) from isolated total RNA on a 15% TBE Urea gel for downstream quantitative PCR.
Byun, JS et al. ELL facilitates RNA polymerase II pause site entry and release. Nature Communications. 2012.In vitro transcribed short hairpin RNA constructs were recovered from 20% denaturing polyacrylamide gels using the ZR small-RNA PAGE Recovery kit. This was used to show that influenza A virus panhandle structure could bind to retinoic acid-inducible gene I (RIG-I) and affect type I interferon production.
Liu, GQ et al. Influenza A Virus Panhandle Structure Is Directly Involved in RIG-I Activation and Interferon Induction. Journal of Virology. 2015.Read More
Need help? Contact Us