Successfully Added to Cart
Customers also bought...
-
 DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the...
DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the... -
 DNA/RNA Shield SafeCollect Swab Collection Kit (1 ml fill) (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA...
DNA/RNA Shield SafeCollect Swab Collection Kit (1 ml fill) (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA...
DNA/RNA Shield DirectDetect
Highlights
- Eliminates the need for RNA (or DNA) extraction, allowing for rapid, direct, cost-effective analysis of samples
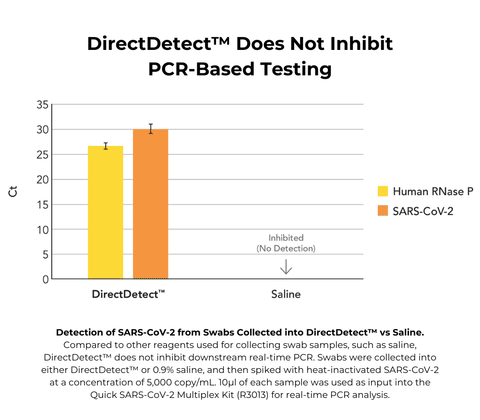
- No inhibition of real-time PCR
- Reduces sample viscosity to minimize pipetting errors with automated liquid handlers
Original Manufacturer
Satisfaction 100% guaranteed, read Our Promise
Innovated in California, Made in the USA
DNA/RNA Shield DirectDetect
Highlights
- Eliminates the need for RNA (or DNA) extraction, allowing for rapid, direct, cost-effective analysis of samples
- No inhibition of real-time PCR
- Reduces sample viscosity to minimize pipetting errors with automated liquid handlers
Original Manufacturer
Satisfaction 100% guaranteed, read Our Promise
Innovated in California, Made in the USA
Description
Performance
Technical Specifications
| Applicable For | RT-PCR, COVID-19 Testing |
|---|---|
| Device Specs | Reagent |
| Reagent Storage | Ambient temperature => 1 year |
| Sample Collection | Swab (1 swab/mL), Saliva (50% v/v) |
| Sample Source | Saliva, oral (and nasal) specimen |
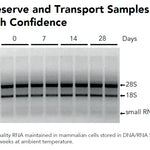
| Sample Stability | Ambient Temperature (4°C-25°C): < 1 week Frozen (<-20°C): Indefinitely |
Resources
Documents
FAQ
No, these are two separate reagents. Once a sample is collected in DNA/RNA Shield it cannot bypass extraction, it must be collected in DNA/RNA Shield DirectDetect in order to work as advertised.
No, DNA/RNA Shield DirectDetect does not inactivate pathogens while DNA/RNA Shield does. Additionally, DNA/RNA Shield DirectDetect preserves nucleic acid for one week, while DNA/RNA Shield preserves RNA for at least 30 days and DNA for at least 2 years.
Reviews
"In India, where testing supplies and vaccinations are difficult to obtain, testing is imperative to diagnosing the SARS-CoV-2 virus. While many biotechnology and testing suppliers have tended to concentrate on their domestic testing efforts, we appreciate Zymo Research's global humanitarian effort to help eradicate COVID-19."
Anu Acharya
Founder and CEO of Mapmygenome
Need help? Contact Us