Successfully Added to Cart
Customers also bought...
-
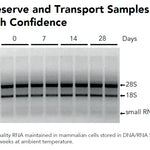 DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the...
DNA/RNA Shield (50 ml)Cat#: R1100-50DNA/RNA Shield reagent is a DNA and RNA stabilization solution for nucleic acids in any biological sample. This DNA and RNA stabilization solution preserves the... -
 DNA/RNA Shield SafeCollect Swab Collection Kit (1 ml fill) (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA...
DNA/RNA Shield SafeCollect Swab Collection Kit (1 ml fill) (1 collection kit)Cat#: R1160The DNA/RNA Shield SafeCollect Swab Collection Kit is a user-friendly collection kit for stabilizing the nucleic acid content of samples collected with a swab. DNA/RNA...
Highlights
- Boost Detection: Included BashingBeads ensure complete lysis of tough-to-lyse samples.
- Ultra-Pure: Ready for qPCR, Next-Gen Sequencing, arrays, etc.
- Simple: Fastest workflow (≤ 20 minutes).
Original Manufacturer
Satisfaction 100% guaranteed, read Our Promise
Innovated in California, Made in the USA
Highlights
- Boost Detection: Included BashingBeads ensure complete lysis of tough-to-lyse samples.
- Ultra-Pure: Ready for qPCR, Next-Gen Sequencing, arrays, etc.
- Simple: Fastest workflow (≤ 20 minutes).
Original Manufacturer
Satisfaction 100% guaranteed, read Our Promise
Innovated in California, Made in the USA
| Cat # | Name | Size | Price | |
|---|---|---|---|---|
| D3004-4-10 | DNA Elution Buffer | 10 ml | $17.00 | |
| D3004-2-50 | g-DNA Wash Buffer | 50 ml | $22.00 | |
| D3004-1-100 | Genomic Lysis Buffer | 100 ml | $72.00 | |
| D3004-5-15 | DNA Pre-Wash Buffer | 15 ml | $12.00 | |
| D6001-3-40 | BashingBead Buffer | 40 ml | $35.00 | |
| C1057-50 | Zymo-Spin III-F Filters | 50 Pack | $71.00 | |
| C1004-50 | Zymo-Spin IC Columns | 50 Pack | $64.00 | |
| C1001-50 | Collection Tubes | 50 Pack | $18.00 | |
| S6012-50 | ZR BashingBead Lysis Tubes (0.1 & 0.5 mm) | 50 Tubes | $121.00 | |
Description
Performance
Technical Specifications
| Applicable For | All sensitive downstream applications such as qPCR and Next-Generation Sequencing. |
|---|---|
| Elution Volume | ≥ 10 µl |
| Equipment | Microcentrifuge, vortex, cell disruptor/pulverizer |
| Processing Time | ≤ 15 minutes |
| Processing Volume | ≤20 mg fungi or bacteria (wet weight), 2x108 bacterial cells, 2x107 yeast cells, or 2x106 mammalian cells |
| Purity | Typical A260/A280 & A260/A230 ≥ 1.8 |
| Sample Source | Fungal and bacterial cell cultures, spores, pollen, nematodes, as well as other microorganisms can also be sampled. |
| Size Range | Capable of recovering genomic DNA sized fragments from up to and above 40 kb. Typical fragment sizes range from 25 kb - 35 kb. If present, parasitic and viral DNA will also be recovered |
| Type | Total DNA |
| Yield | ≤ 5 µg total DNA |
Resources
Documents
FAQ
A viscous sample can indicate incomplete sample lysis. Try using less of your sample and optimize bead beating conditions (duration, speed, time) to ensure samples are thoroughly lysed. After bead beating, pellet the cell debris before moving on. Adding more Genomic Lysis buffer to the lysate can help dilute and deproteinate the sample, making the sample less viscous and more suitable for DNA recovery.
We have validated our kits with both high-speed homgenizers and low-speed disruptors. We highly recommend users to optimize their bead beating conditions for their own instruments. We recommend using a 2 ml-tube adapter to ensure that the bead beating is efficent (do not use adapters made of foam). For high-speed homogenizers, we recommend a total of 5 mins bead beating (1 min interval at 6.5 m/s with 5 mins rest, repeat 5 times). For low-speed cell disruptors, we recommend 30 mins at max speed.
Addition of beta-mercaptoethanol is recommended to enhance sample lysis, but can be substituted with dithiothreitol (DTT, final concentration of 10 mM). However, if bead beating is optimized and lysis is efficient, the addition of BME is not necessary and can be omitted.
No additional RNase A treatment is required when processing samples within kit capacity. The selective chemistry allows for binding of double stranded DNA to the column and for RNA to flow through.
Citations
Need help? Contact Us